MOT revealed one of the most admiring qualities of bonded atoms. In a molecule atoms are ready to share the burden of other atom. You will understand it better by an example of (CO3)-2 ion. This ion has got extra stability by team work of its atoms. Let’s see how these atoms work together to gain extra stability.
 When you work out the Lewis dot structure for (CO3)-2ion you will find that two of the three Oxygen atoms are negatively charged (O-1). Write the configuration of C and O atoms.
When you work out the Lewis dot structure for (CO3)-2ion you will find that two of the three Oxygen atoms are negatively charged (O-1). Write the configuration of C and O atoms.Electronic configuration of 6C: 1s2, 2s2, 2p2
Electronic configuration of 8O: 1s2, 2s2, 2p4
When O atom gains one electron it will form O-1 ion so the
Electronic configuration of O-1: 1s2, 2s2, 2p5.
Thus 6+8+9+9=32 electrons are involved in the formation of (CO3)-2 ion. With the help of VSEPR we can predict the shape of the molecule.
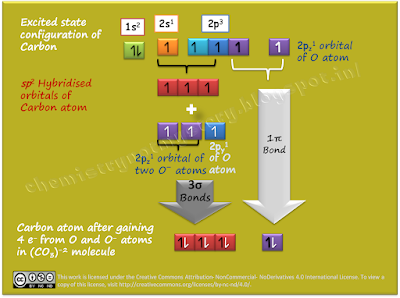
Excited state configuration of central atom C is: 1s2, 2s1, 2p3
It opts sp2 hybridization and uses three sp2 hybridised orbitals for making three sigma (σ) bonds and unhybridised pz orbital for making one pi (π) bond. That’s why its shape is triangular planer. You can see here that one of the three O atoms has double bond and two O atoms have to bear negative charge. But MOT finds out the real picture of (CO3)-2ion.
We have counted that (CO3)-2 ion has 32 electrons. We are not able to distribute them in MOs because the energy order of MOs for (CO3)-2ion isn’t known exactly. That’s why MOT suggests a bypass to calculate the bond order of such multi cantered (atomic) molecules. This procedure involves the following steps:
- Find out the shape by using VSEPR: triangular planer
- Add up the total number of valence electrons: C has 4 valance electrons, O has 6 and O-1 has 7, thus, the total 4+6+7+7=24
- Determine the number of electrons that participate in the formation of (π) bonds: = Total number of valence electrons – (total number of electrons involved in (σ) bonds)+(lone pair of electrons): (CO3)-2has 3 (σ) bonds which involved 6 electrons and each O has two sets of lone pairs thus 6 sets that means 12 electrons, now we have to subtract 6+12=18 electrons from total number of valence electrons (24), so we get 24-18= 6 electrons which will participate in π bonding.

- Count the number of atomic orbitals which can take part in π bonding: C has one 2pz orbital and each of three O has one 2pz orbital thus 4 AO are available for π bonding.
- Combine these AO and find out the number of MO which will be formed: 4 AOs combined to form 4 four centered MOs as (CO3)-2ion is a four centered molecule. That means MOs are spread over all the four atoms.
- Decide which MO are bonding, antibonding and non bonding: lowest energy MO will be bonding and highest energy MO will be antibonding and remaining two will be non bonding and also of same energy. Non bonding MO is intermediate state of bonding and antibonding.
- Find the number of π bonds: on distributing 6 electrons in MO you will find that 2e are filled in bonding MO and 4e are filled in nonbonding MO. On applying the formula used to calculate bond order we will get (2 bonding e) - (0 antibonding e) /2 = 1 π bond.

By VSEPR we know that (CO3)-2ion has 3 (σ) bonds and MOT gives the number of π bond formed. But the important discovery from MOT is about the distribution of π bond. MOT says that this π bond is equally distributed in between all atoms. That means each of the three C-O bonds has got 1/3 share of π bond. Thus each C-O bond will possess the (1+1/3) bond order.
That means the pair of electrons of π bond is not localised, it is delocalised over all bonds. So, all the bonds share the burden and give extra stability to the molecule. But how can we draw the bond of fractional bond order? In the next post we will try to solve this problem.

No comments:
Post a Comment